

Compliant with healthcare regulations
Bleepa® is compliant and secure, making it easy to acquire and put into practice for your team. It can be easily integrated into any existing IT infrastructure. We support standard interfaces for a smooth implementation and integration.

Zero-footprint application
Bleepa® is a secure, encrypted, zero-footprint application, meaning that no patient data is stored locally on any device.
- Manufactured using processes that adhere to security standards
- UKCA accredited communications platform
As a UKCA marked medical device, Bleepa® is certified to display clinical images at a suitable quality for clinical review, ensuring patient safety and reducing inadvertent risk for clinicians and their employers.
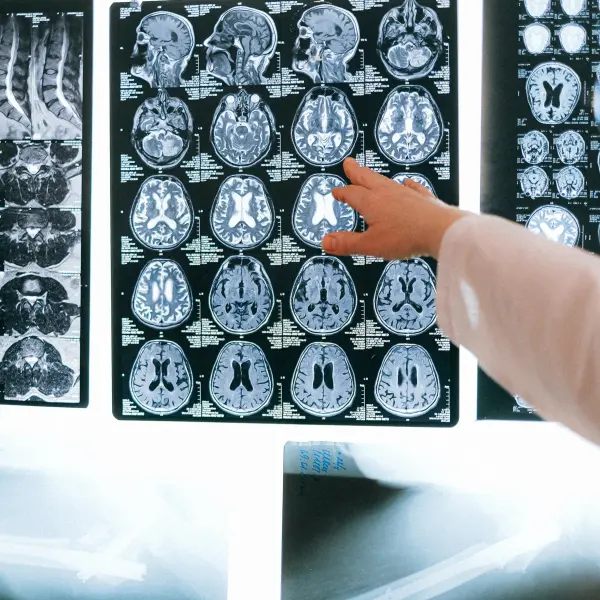
Certified medical device
As a communications platform certified as a medical device for clinical results display, Bleepa® enables providers to achieve compliance with the Information Commissioner’s Office (ICO), Care Quality Commission (CQC) and the Medicines and Healthcare products Regulatory Agency (MHRA) requirements for clinical communication.
With Bleepa®, clinicians can collaborate at any time, whilst having a common view of a patient and access to medical images, test results and other documents in one safe, secure platform, both on and offsite. Discussions can happen flexibly on a fully auditable platform that captures them as part of the patient record, reducing the documentation burden for clinicians and ensuring a complete, integrated record is maintained.

Meeting regulatory requirements
There are several regulatory requirements that NHS organisations need to comply with regarding clinical communications and patient records, all of which Bleepa® complies with for peace of mind for those organisations and their IT and governance teams.
Use of non-compliant social media apps
Using standard messaging apps creates information governance concerns and breaches clinical safety regulations, resulting in a liability risk for clinicians and their employing organisation. Following the Information Commissioner’s Office (ICO) ruling, it is imperative that healthcare providers take active steps to move their clinicians onto compliant communication platforms.

Care Quality Commission (CQC) requirements
Healthcare organisations have a requirement to provide appropriate tools for facilitating clinical service delivery which includes communication platforms.
A communication platform used by frontline clinicians needs to write back to the patient’s medical record in order to maintain a ‘contemporaneous’ record (existing at the same time) for the patient and to maintain clinical safety and reduce medico-legal risks. (CQC Regulation 17(2)(c))
Medical device regulation
The MHRA requirement is that the use of digital images, such as photos or radiology images, for clinical decision making requires any platform involving them to be fully certified as a medical device. This is a matter of clinical safety to ensure that images are displayed in the right quality to avoid details being missed that could lead to the harm of the patient.

Our accreditations
- We exceed the standards for the NHS Data Security and Protection Toolkit and are aligned with the NHS Digital Technology Assessment Criteria.
- We are certified to the Medical Device Manufacture Quality Standard, ISO 13485. Our Quality Management System (QMS) incorporates the UK requirements for medical device manufacture to allow us to produce UKCA marked products.
- We are registered as a Class A medical device with the Central Drugs Standard Control Organisation (CDSCO) in India.
- We have ISO 27001 certification for information security, cyber security and privacy protection. ISO 27001 is the international standard that outlines the requirement for an information security management system.
- Bleepa® is a UKCA marked communications platform incorporating medical image display, actively in use by the NHS.









